


Next: About this document ...
Up: Segmentation of Progressive Multifocal
Previous: Segmentation of Progressive Multifocal
- 1
- Johnston L, Atkins MS, Mackiewich B,
Anderson M. Segmentation of Multiple-Sclerosis Lesions in Intensity
Corrected Multispectral MRI. IEEE Trans Med Ima 1996;15(2):154-69
- 2
- Zijdenbos AP, Dawant BM. Brain Segmentation
and White Matter Lesion Detection in MR Images. Critic Rev Biomed Eng
1994;22(5-6):401-65
- 3
- Filippi M, Yousry T, Baratti C, et al.
Quantitative assessment of MRI lesion load in multiple sclerosis. A
comparison of conventional spin-echo with fast fluid-attenuated
inversion recovery. Brain 1996;119(Pt 4):1349-1355
- 4
- Hajnal JV, Oatridge A, Murdoch J, Kasuboski L,
Bydder GM, Failure of FLAIR sequences to suppress CSF in the Posterior
Fossa: Diagnosis of a Problem and Solution with adiabatic inversion
pulses. In Proc. 6th Annual Meeting of the International Society for
Magnetic Resonance in Medicine, p. 1350, Sydney, April 18-24, 1998
- 5
- Filippi M, Gawne-Cain ML, Gasterini C, et al. Effect of training and different measurement strategies on the
reproducibility of brain MRI lesion load measurements in multiple
sclerosis. Neurology 1998;50(1):238-244
- 6
- Mark AS, Atlas SW. Progressive multifocal
leukoencephalopathy in patients with AIDS: appearance on MR images.
Radiology 1989;173(2):517-520
- 7
- Trotot PM, Vazeux R, Yamashita HK, et
al. MRI pattern of progressive multifocal leukoencephalopathy (PML)
in AIDS. Pathological correlations. J Neuroradiol 1990;17(4):233-254
- 8
- Bastianello S, Bozzao A, Paolillo A, et al. Fast spin-echo and fast fluid-attenuated inversion-recovery
versus conventional spin-echo sequences for MR quantification of
multiple sclerosis lesions. Am J Neuroradiol 1997;18(4):699-704
- 9
- Yousry TA, Filippi M, Becker C, Horsfield MA,
Voltz R. Comparison of MR pulse sequences in the detection of multiple
sclerosis lesions. Am J Neuroradiol 1997;18(5):959-963
- 10
- Gawne-Cain ML, Silver NC, Moseley IF, Miller
DH. Fast FLAIR of the brain: the range of appearances in normal
subjects and its application to quantification of white-matter
disease. Neuroradiology 1997;39(4):243-249
Figure 1:
Automated extraction of a medium-sized
lesion in two scans acquired consecutively with two head orientations
(top: First scan; bottom: Second scan). Crosses on the left indicate
several manually-selected seed points, which, given individually as
input to the automated algorithm, all yielded the segmented result
shown on the right.
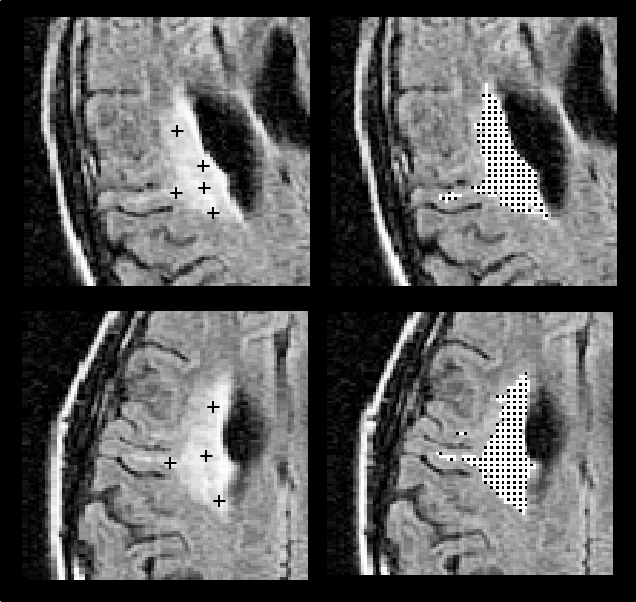 |
Figure 2:
(a) Neighborhood used during flooding:
the region will grow into volume elements (voxels) with intensities
above a threshold 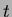 and adjacent to a voxel already known to
belong to the lesion (shown in gray), on the same slice as well as
on the two adjacent slices. This local flooding is applied
recursively until no neighbors above
and adjacent to a voxel already known to
belong to the lesion (shown in gray), on the same slice as well as
on the two adjacent slices. This local flooding is applied
recursively until no neighbors above 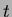 can be found. (b) The
threshold
can be found. (b) The
threshold 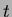 is adaptively determined for each lesion, by starting
from the intensity value at the manually selected seed point, and
progressively decreasing the threshold by discrete amounts
is adaptively determined for each lesion, by starting
from the intensity value at the manually selected seed point, and
progressively decreasing the threshold by discrete amounts 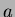 ,
until the ratio of flooded lesion volumes obtained for
,
until the ratio of flooded lesion volumes obtained for 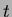 and
and 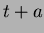 becomes greater than a given constant
becomes greater than a given constant 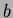 . This typically ocurs as
the lesion volume explodes when the threshold becomes sufficiently
low as to include voxels in the normal white matter.
. This typically ocurs as
the lesion volume explodes when the threshold becomes sufficiently
low as to include voxels in the normal white matter.
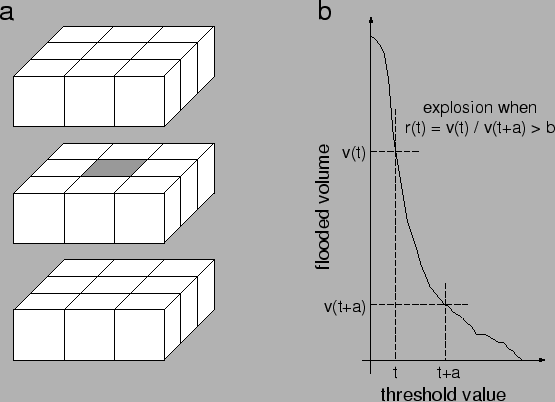 |
Figure 3:
Results of the analysis. (a): Lesion
volume computed with the automated algorithm correlated well with the
volume found with the manual outlining method. (b): However, the
difference between manual and automated volumes was often large,
particularly for small lesions (for which an error of only a few
pixels can be significant). (c)-(e): Experiments with pairs of
repositioned scans. Correlation of computed lesion volumes between
scans 1 and 2 was excellent for the automated method (c), and also
very good for the manual method (d). Volume differences were, however,
much lower with the automated method (e; black circles) than with the
manual method (e; triangles).
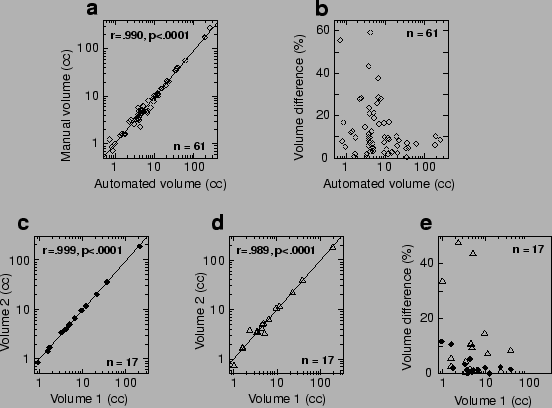 |
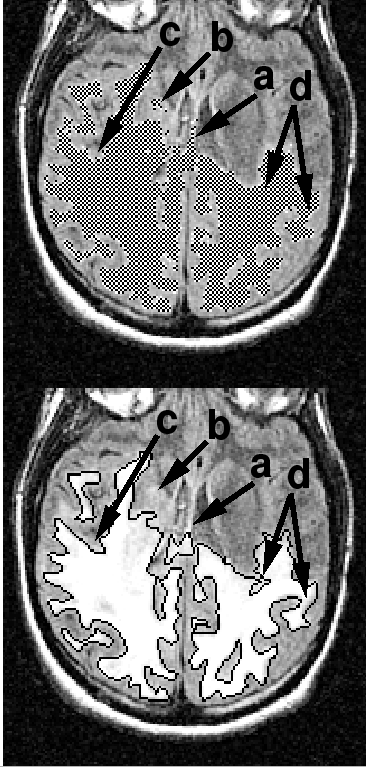
Figure 4:
Major sources of discrepancies between the
automated (top) and manual (bottom) methods: (a) Imaging
artifacts: Hyperintensities at the brain/fluid interface are ambiguous
for the automated algorithm; (b) 3D shape coherence: Here the
human observer omitted a small island connected to the main body of
the lesion in another slice; (c) Small shape irregularities: The
manual drawing smoothed out the exact shape of the lesion, which was
correctly followed by the automated algorithm; and (d)
Inconsistent drawing rules in the manual method, more conservative in
some regions (left arrow) than others (right arrow).
 |



Next: About this document ...
Up: Segmentation of Progressive Multifocal
Previous: Segmentation of Progressive Multifocal
Laurent Itti
2001-04-03




